Developing novel neuromodulation technologies to enable real-time, precise excitation or inhibition of a particular neuron or neural network is vitally important for in-depth interpretation of human neurology and the exploration of new treatments for neurological diseases. Biological scaffolds (bio-scaffolds) play an extremely important role in biomedical engineering technologies such as tissue repair and disease treatment.
Mainly providing mechanical support, conventional bio-scaffolds demonstrate very limited biofunctionality. As a result, biochemicals such as growth factors and drugs are often used to potentiate their bioactivities. However, the in vivo release of biochemicals is faced with problems such as weak controllability, low spatial and temporal resolutions, and short duration. As a comparison, biomodulation based on physical fields (such as optoelectronic signals) exhibits controllable and durable operations with high spatial and temporal resolutions, which earns itself a broad application prospect in the field of biomodulation.
Fig. 1. Conceptual illustration of the 3D optoelectronic scaffold
Associate Professor Xing Sheng and his team from the Department of Electronic Engineering at Tsinghua University recently reported a new outcome in this field. Working together with other research teams, they developed a bioactive optoelectronic scaffold. The researchers integrated thin-film monocrystalline silicon structures with organic biomaterials, and used nanoscale processing to fabricate a three-dimensional, multilevel optoelectronic bio-scaffold that mimics natural bone tissue. When illuminated, the Si-based monocrystalline films can generate electrical signals that stimulate and regulate the activities of bone marrow mesenchymal stem cells (MSCs), thereby further promoting the repair and regeneration of animal cranial defects. Compared to conventional electrical stimulation, this wireless optoelectronic approach reduces damage to surrounding tissues caused by wire connections. In addition, as the thin-film Si-based scaffold safely degrades inside living organisms, presenting desirable biocompatibility, it can help avoid the need for secondary surgeries and reduce the risk of infection. Such a biodegradable and wirelessly powered optoelectronic scaffold offers a new strategy for studies related to tissue engineering and clinical applications.
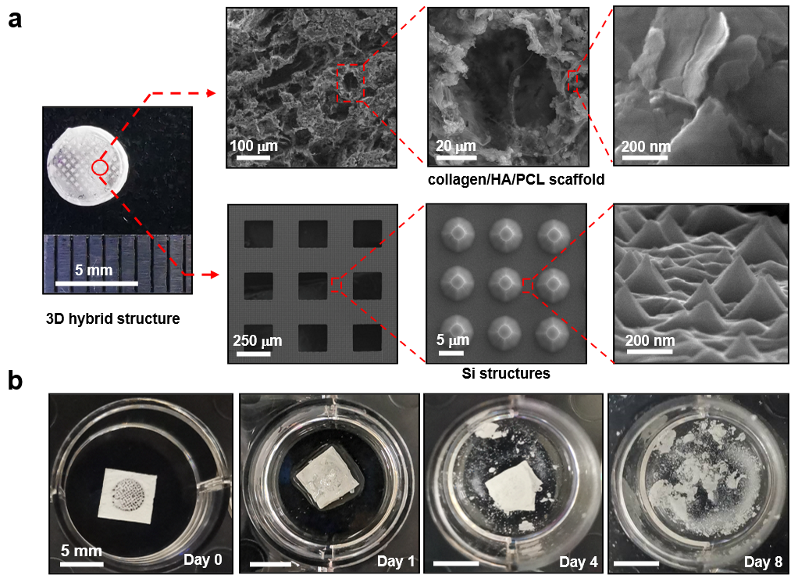
Fig. 2. Structures and degradation properties of the 3D biomimetic optoelectronic scaffold
The research team integrated thin-film Si-based structures with bone marrow MSCs and biological tissues to build optoelectronic biointerfaces. Without genetic encoding techniques such as optogenetics, researchers achieved effective modulation of physiological activities, such as membrane potentials, intracellular calcium dynamics, and cell proliferation and differentiation, by means of polarized photo-induced electric fields generated at the silicon-cell interface under illumination.
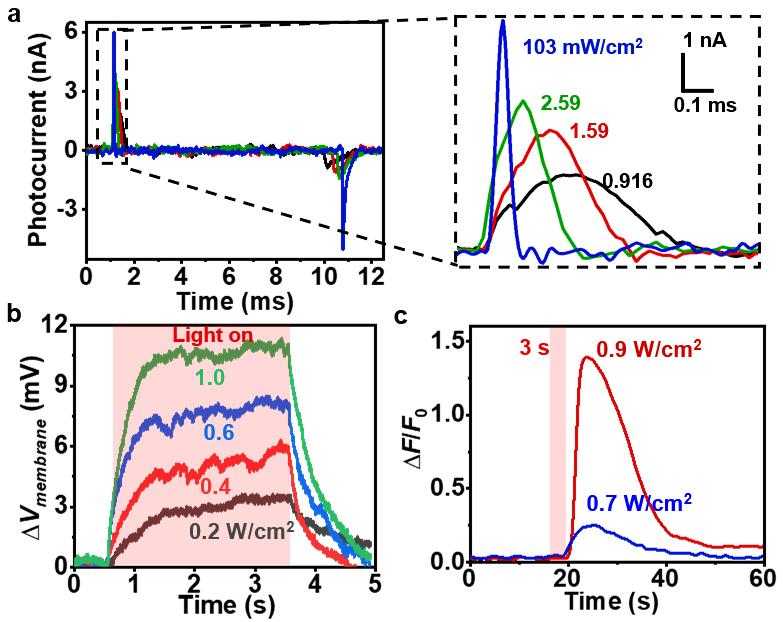
Fig. 3. Optoelectronic reponses of Si thin films and Optoelectronic modulation on cell membrane potentials and calcium ion concentration
Researchers then integrated Si-based monocrystalline films with biomaterials such as hydroxyapatite, to fabricate a 3D bone-like bio-scaffold, which exhibits optoelectronic responses because of the Si films. In in vivo animal experiments, the 3D optoelectronic bio-scaffold was implanted into the defect site in rat skulls. Facilitated by optoelectronic stimulations, the scaffold effectively promoted the repair and regeneration of cranial bone tissue, which confirmed and validated the application of optoelectronic bio-scaffolds in the biomedical field.
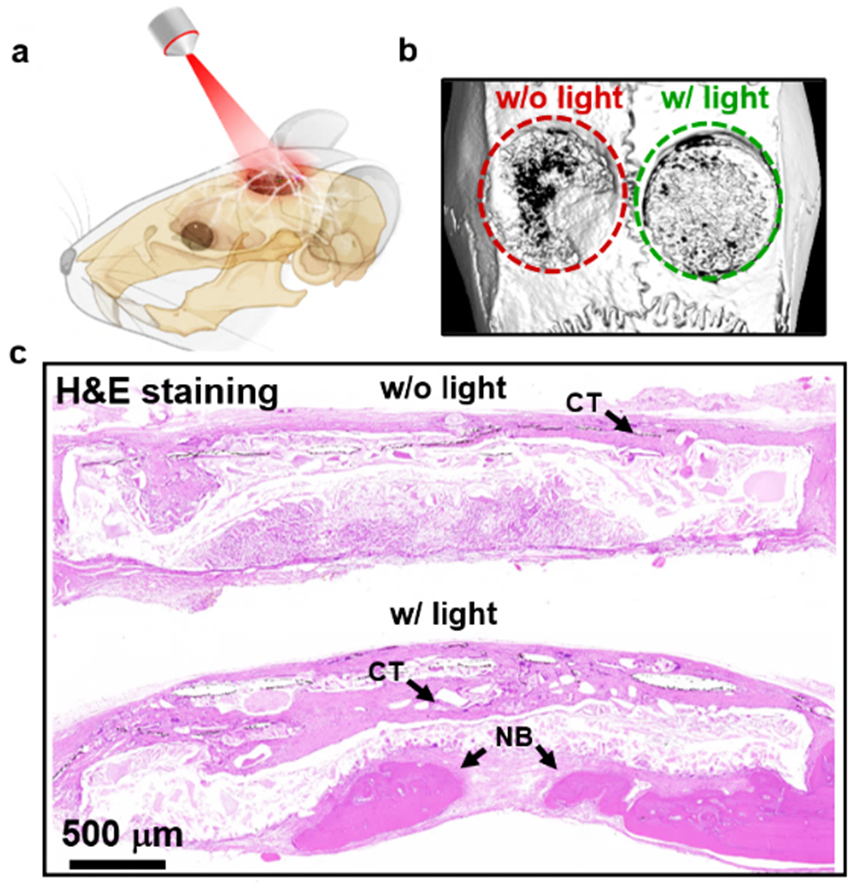
Fig.4. Repair of cranial defects in a rat model by the 3D optoelectronic bio-scaffold
The research outcome was published in Science Advances, titled "A 3D Biomimetic Optoelectronic Scaffold Repairs Cranial Defects".
The corresponding authors of this paper include Associate Professor Xing Sheng from the Department of Electronic Engineering and the IDG/McGovern Institute for Brain Research at Tsinghua University, and Associate Chief Physician Yuguang Wang from the Peking University School of Stomatology. Huachun Wang, a PhD student from the Department of Electronic Engineering, is the first author. The joint research team also included members from the Tsinghua School of Materials Science and Engineering, the School of Life Sciences, the Peking Union Medical College Hospital, the Peking University Hospital of Stomatology, and Jishuitan Hospital. The research was supported by the National Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Self-Initiated Research Program of Tsinghua, the Self-Initiated Research Program of the Department of Electronic Engineering, and the State Key Laboratory of New Ceramic and Fine Processing of Tsinghua University, among others.
Access full-text paper at https://www.science.org/doi/10.1126/sciadv.abq7750